The Electrotonic Workbench operates in the following manner.
1. The anatomy and biophysical properties of the cell are
specified by the user through any of the usual data entry
mechanisms (i.e. read from files, created algorithmically, entered
or edited manually, or any combination thereof).
2. Since attenuation depends on direction of signal propagation
[Tsai et al. 1994],
NEURON computes the voltage attenuations along each branch of
the model for signals propagating away from (Vout) and toward
(Vin) a user-selected reference point.
The reference point, which can be any functionally or anatomically relevant
location in the cell, may be selected via NEURON's graphical interface.
Attenuation is a function of frequency, so it is often informative to compute
a pair of Vout and Vin transforms at several frequencies
of interest.
Because voltage attenuation in one direction is the same as current or charge
attenuation in the opposite direction, the Vout and Vin transform
pairs provide a complete picture of the electrotonic architecture of the cell.
3. Graphical renderings of the Vout and Vin transforms
are generated, in either the "neuromorphic" or "L vs. x" [O'Boyle et
al. 1996] format. The neuromorphic renderings, which we
demonstrate in this abstract, give the quickest intuition to the
electrotonic architecture of a neuron.
These redraw the cell in a way that preserves its overall shape, but with
the distances between adjacent measurement points proportional to the
logarithm of voltage attenuation between them. Since attenuation depends
on direction of signal propagation [Tsai et al. 1994], each anatomical branch
has two representations in electrotonic space depending on which of its ends
is "upstream" from the other. The proximal ends are "upstream" in the
Vout transforms (centrifugal propagation of voltage),
whereas the distal ends are "upstream" in the Vin transforms
(centripetal propagation of voltage).
4. Numeric results are available for subsequent quantitative
analysis and can be written to output files as appropriate.
Computations and graphical renderings are all handled in an integrated fashion because the Electrotonic Workbench runs within the NEURON environment. This means the user can examine the results immediately and is not bothered with details such as moving intermediate data files between different programs. Furthermore, all operations are customizable and under program control, so the user can automate a series of analyses that iterate over a range of frequencies, biophysical parameters, anatomical dimensions, or combinations thereof.
For the purpose of illustration, we show the transforms of the neocortical pyramidal cell whose morphometric data accompanies the standard NEURON distribution (($NEURONHOME)/nrn/demo/pyramid.nrn). The biophysical parameters we used are Ra = 200 ohm-cm, Rm = 14,500 ohm-cm^2, Cm = 1 uf / cm^2, with a uniform spine density that adds 1.25 um^2 per um length of neurite [Cauller and Connors 1992]. The transforms and their graphical renderings were performed with NEURON executing the Electrotonic Workbench under MS-Windows 3.1 on a 90 mHz Pentium PC with 16 MB RAM. Computation and display of the Vout or Vin transform was complete within 1 second for this model cell with 79 segments. All images were captured directly from the VGA display using HiJaak Pro, and subsequently composed and labeled with HiJaak Draw (HiJaak Graphics Suite v. 3.0, Inset Systems, Inc.).
Observations described below are similar to findings that we have reported elsewhere in hippocampal pyramidal neurons [Carnevale et al., 1995b].

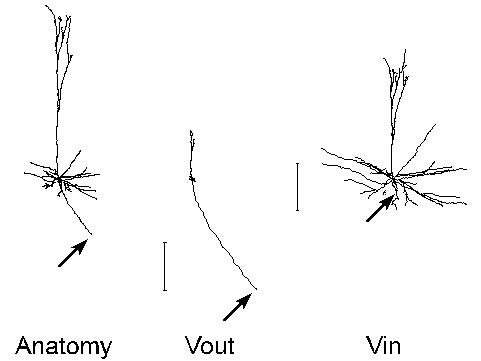
On the left is a side view of the anatomy of the cell. The center and right images are the neuromorphic renderings of the Vout and Vin transforms relative to the soma at 0 Hz. The calibration bars are in "log attenuation" units (one unit of separation in electrotonic space corresponds to an e-fold decay of voltage). Because the soma is the "reference point" for the transforms shown in this and the following figure, electrotonic distance is to be measured away from the soma in the Vout image, and toward it in the Vin image.
Note that:

Attenuation increases rapidly with frequency, as these transforms demonstrate. Consequently, at 40 Hz the spatial scope of action for signals generated in the soma or dendrites would be very limited (i.e. localized processing predominates). Based on this electrotonic architecture, one would expect that synaptic inputs responsible for network synchronization at high frequency should be located quite near the soma. Modeling studies [Lytton and Sejnowski 1991] and experimental observations [Ylinen et al. 1995] support this prediction in hippocampus.
A somatic reference point is the most logical choice when one is concerned with issues such as
This is clearly seen by comparing the central image (Vout) of
the following figure
(dendritic reference location D indicated by arrow)
with the Vout and Vin transforms in the first figure
(Electrotonic architecture at 0 Hz: somatic reference).

Likewise, along this path the Vin transform relative to D is the same as the Vout transform relative to the soma, but elsewhere the Vin transforms are identical (compare right image of this figure with the Vout and Vin transforms in the first figure (Electrotonic architecture at 0 Hz: somatic reference).
Since the "reference point" for the transforms shown in this figure is dendritic location D (arrow), electrotonic distance is to be measured away from D in the Vout image, and toward D in the Vin image. For the sake of clarity, D was placed on a termination, but any other nonsomatic location could have been used.
Address correspondence to:
N.T. Carnevale
Psychology Dept.
Yale University
P.O. Box 208205
New Haven, CT 06520-8205
phone 203-432-7363
fax 203-432-7172
email ted.carnevale@yale.edu
Page and images Copyright © 1996, 1999 N.T. Carnevale. All RightsReserved.